[Photo of courtesy of Doctors Without Borders]
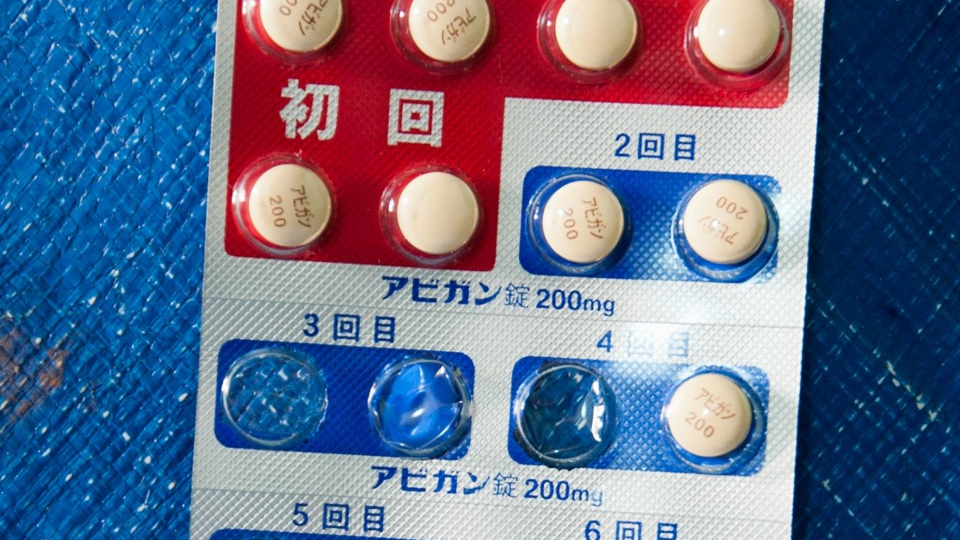
The central Japanese prefecture of Toyama will help boost production of antiviral flu drug Avigan, which is seen as effective in treating COVID-19, the respiratory disease caused by the new coronavirus, its governor said.
“I will ask pharmaceutical companies in Toyama Prefecture to cooperate in boosting production (of Avigan),” Toyama Gov. Takakazu Ishii said recently. “If the made-in-Toyama medicine can help Japan and the rest of the world (in their fight against the coronavirus), we will do whatever we can.”
Japan plans to offer the drug, developed by Fujifilm Toyama Chemical Co., a subsidiary of Fujifilm Holdings Corp., for free to at least 20 countries hoping to use it to treat coronavirus patients. The Japanese government also plans to triple the stockpile of Avigan for use in treating 2 million COVID-19 patients.
Fujifilm Toyama Chemical is headquartered in Tokyo but has plants in the central Japan prefecture, known as a major producer and seller of medicine in the country.
Kimiyasu Shiraki, a professor emeritus at the University of Toyama, is among researchers involved in the development of the drug, also known as Favipiravir.
Related coverage:
Japan to offer free anti-flu Avigan drug to 20 virus-hit nations
Japan PM Abe declares state of emergency amid widespread virus infections
A key ingredient of the antiviral drug has been imported from China, but supply is recently stalled due to the global spread of the virus.
“A certain number of facilities in Toyama Prefecture can respond to the call for production increase,” Ishii said.
As domestic production of the ingredient instead of using imports from China means more costs to produce Avigan, the governor said, “I will ask the central government to support capital investment among others.”
Researchers at Wuhan University and other institutions in China have said the drug was effective on coronavirus patients, especially for those with mild symptoms of COVID-19.
Japan started a clinical trial of Avigan for COVID-19 patients in late March, while Israel and the United States will also start such a trial, according to the Israeli government and Fujifilm Holdings.
Avigan was approved for manufacture and sale in Japan in 2014 for use against novel or re-surfaced influenza virus infections. Expectant mothers or women who might become pregnant cannot take the drug due to the risk of birth defects.